product description
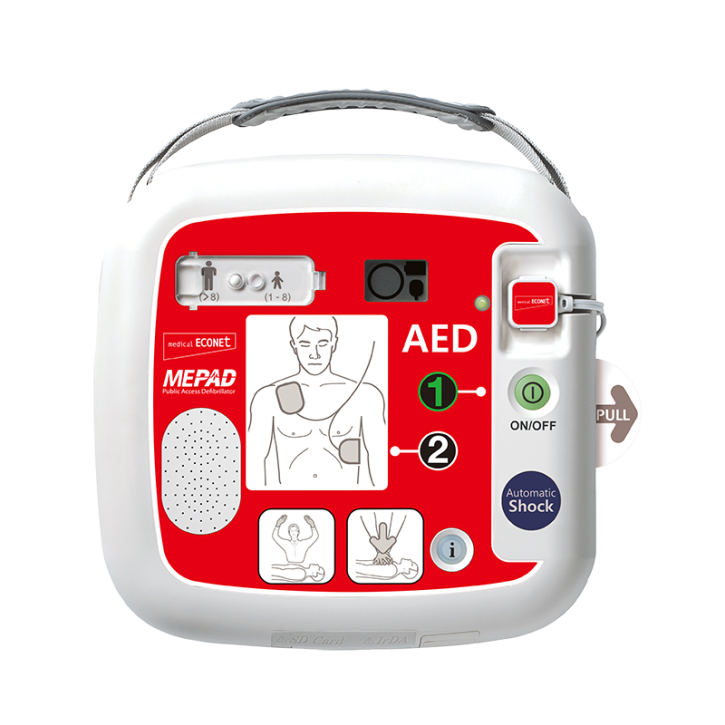
AED Defibrillator ME PAD auto - Fully automatic defibrillator for adults and children
The ME PAD was specially developed for use by laypersons. Clearly understandable spoken instructions and pictograms guide the rescuer through the resuscitation.
• Fully automated defibrillator
• Fully automatic shock delivery by the device
• Automatic adjustment of the volume of the voice announcements to the environment
• Emergency switching to children under 25 kg possible without changing electrodes
• Automatic power switching when connecting children's electrodes
• Automatic self-test and display of readiness
• Internal quality monitoring of the electrode pads
• All relevant data of the last 5 reanimations are saved
• Always up to date with simple software updates
• Rugged design / US Army IP55 and MIL-STD 810G
• 5-year manufacturer's guarantee
• Software according to ERC 2015
• 16 different languages selectable
Technical Details:
Dimensions: 260 x 256 x 69.5 mm (W x L x H)
Weight: 2.4 kg including batteries and electrodesBattery: Lithium battery for standby operation up to 5 years or 200 shocks
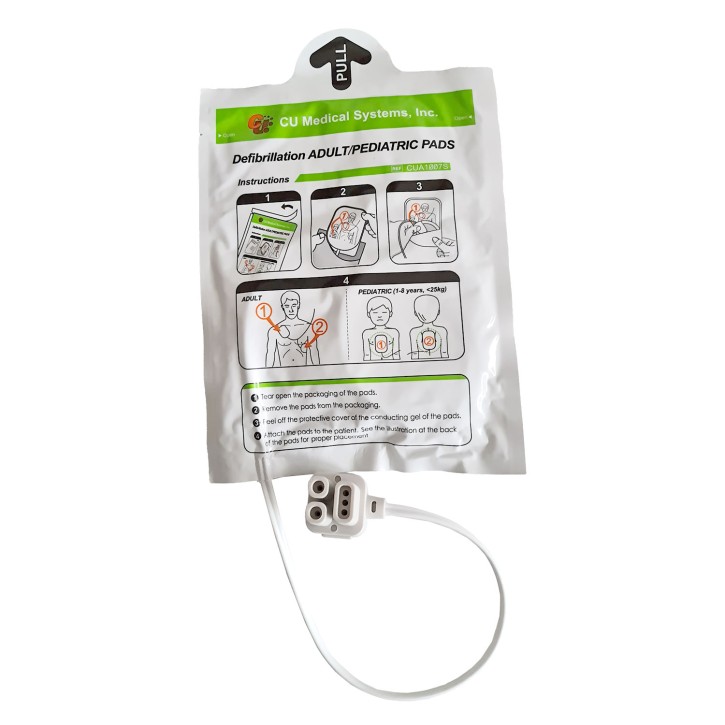
The scope of delivery includes the battery, electrode PADs for adults and a transport bag.
The device instruction required in Germany according to MPBetreibV § 10 Section 1 is not included in the purchase price.
Info: In Germany, defibrillators are subject to the Medical Devices Operator Ordinance, the MPBetreibV. Accordingly, a defibrillator may only be operated in Germany if the necessary authorization has been granted (can be read in MPBetreibV § 10 Section 1). In Germany, this authorization is regulated by a device briefing, which may only be carried out by the manufacturer himself or by a person authorized by him.
The following happens during the briefing:
- The defibrillator will be functionally tested and commissioned at the site;
- The proper handling, use and maintenance of the defibrillator will be instructed, checked and documented.
The instruction obligation does not apply if
- You already have an identical defibrillator (same manufacturer & same model); or
- The defibrillator is intended for a private household and is not made available for other people.
Please note that it is your duty as the operator and not that of the supplier to have such instruction carried out before the first use.
You will also find suitable accessories such as Battery, electrode pads, wall bracket and extremity electrodes.
The safety check (STK) at 2-year intervals that has been mandatory in Germany for most defibrillators since January 1st, 2017 is not included in the purchase price.
Of course we are happy to offer you instruction and STK. Please ask!
Please contact us for more information and an offer by e-mail or via our Contact form.